World’s first artificial pancreas is a turning point in the treatment of type 1 diabetes
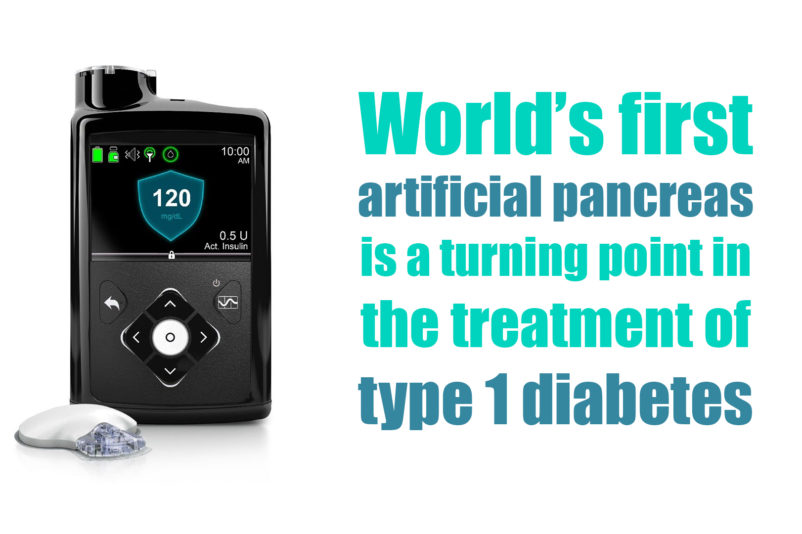
US Food and Drug Administration (FDA) officially announced the approval of the first artificial pancreas for patients with type 1 diabetes. Medtronic’s MiniMed 670G hybrid closed looped system is the first device that automatically tracks the blood glucose (sugar) concentration. Based on the received data it provides the required basal insulin doses. The device is suitable for patients 14 years of age and older, diagnosed with type 1 diabetes.
Aspects of Physiology & Pathophysiology
The pancreas is an organ of the endocrine system, which naturally produces and supplies a low, continuous rate of insulin. This rate is known as basal or background insulin. The hormone regulates the metabolism of carbohydrates (mainly), fats and protein. It assists in the absorption of glucose from the blood into fat, liver and skeletal muscle cells. In these tissues the absorbed sugar is converted into either glycogen or fats. It also affects the synthesis of proteins in a wide variety of tissues.
The pancreas of the patients with diabetes, has lost the ability to produce or respond to insulin. Approximately 5 percent of people with diabetes have the type 1. It is also known as juvenile diabetes, and it is typically diagnosed in children and young adults. In their cases the pancreas does not produce insulin, and they have to consistently monitor their glucose levels throughout the day and also have insulin injection, in order to avoid becoming hyperglycemic (high glucose levels). The proper treatment of type 1 diabetes should also include a healthy eating plan and physical activity.
The importance behind this revolutionary device
The “artificial pancreas” provides people with type 1 diabetes greater freedom to live their lives without having to consistently and manually monitor baseline glucose levels and administer insulin. Unlike previous models of Medtronic’s device, which registers only the abnormally low levels of sugar in the blood, MiniMed 670G registers both low and high levels. It also adjusts the level of the hormone with little or no input of the user.
The device measures glucose levels every five minutes and automatically administers or withholds insulin. The system includes a sensor that attaches to the body and measures glucose levels under the skin; an insulin pump strapped to the body; as well as an infusion patch connected to the pump with a catheter that delivers insulin. This is the way the device automatically adjusts insulin levels and is introduced as world’s first “artificial pancreas”. Still, users should manually request insulin doses to counter dietary carbohydrate consumption. For this reason, the system is called a “hybrid” one.
FDA’s approval of the revolutionary device is a result of the data gathered from a preliminary study conducted among 123 patients with type 1 diabetes aged between 14 and 75 years. The clinical trial consisted of an initial two-week period where the system’s hybrid closed loop was not utilized. It was then followed by a three-month study during which trial participants conducted glycemic control with 670G for over 3 months. As a result, the clinical research professionals have reported a decrease of both hypoglycemic and hyperglycemic episodes, but also reduced variations in serum glucose levels.
In addition, the clinical study showed decreased glycosylated hemoglobin levels from 7.4% to 6.9%, while the glucose concentration recorded by the sensor was in 70% of the cases in the targeted amount. No serious adverse events, episodes of diabetic ketoacidosis (DKA) or severe hypoglycemia (low glucose levels) were reported during the trial. Nevertheless, the risks associated with the utilization of the device may include hypo- or hyperglycemia and skin irritation around the 670G’s infusion patch.
FDA regards the version of this device unsafe for children aged 6 years or younger and in patients who require less than eight units of insulin per day. Medtronic is currently performing clinical studies, which will evaluate the safety and effectiveness of the system in children with diabetes aged between 7-13 years. FDA is also requiring a post-market study. It will deliver more clarity of how the device performs in real-world settings.
Successful results are achieved after good clinical trial management
The breakthrough moment for the appearance of the artificial pancreas is a result of the continuous and systematic efforts of clinical research and medical device professionals. The use of a clinical trial management systems to manage successfully clinical trials in clinical research, results in the achievement of such milestones that we are witnessing today. The good results behind the conduction of any clinical studies is in the good management.
As an effective and lightweight clinical trial management system, Clinicubes offers integrated solutions for every single aspect and phase of clinical studies. In its core, the CTMS is systematized, well-built and easy-to-use. Clinicubes provides with an easier way for collecting, retaining and archiving patient and scientific data. Clinical research conductors can also track deadlines, schedule visitations and monitor treatment progress. It also increases the productivity of the clinical research site and the number of successfully completed trials.
Become our partner
We are currently looking for partners for the implementation and customization of Clinicubes. Get in touch and take advantage of what our clinical trial management system offers.
You can find a PDF version of this article here: World’s first artificial pancreas
Official Information: FDA
Picture Source: Medtronic




