The most recently conducted genetic study signalizes that the accumulation of abdominal fat is a major risk factor for development of type 2 diabetes and coronary heart disease (CHD). In particular, the research points that individuals with a...
Blog
Visit our blog to discover the latest news, updates and posts about Clinicubes, the CTMS market and the clinical research industry
Xarelto (rivaroxaban) is the first and only non-vitamin K antagonist oral anticoagulant. The drug is currently under assessment among high risk patients with coronary or peripheral artery disease. The major news announced by Bayer and Janssen (the developers Xarelto) is that the primary end point of the Phase III COMPASS study of the clinical trial has been met earlier. More importantly, the results are “overwhelmingly” satisfying. The medicine has shown positive safety and...
Drug approvals are the aimed goal of clinical trials so that they can positively change people’s lives and treat what have been considered incurable. Unfortunately, 2016 has been unsuccessful for newcomers since only 22 medicines have been approved by the U.S. Food and Drug Administration (FDA). The hopes of the drug-makers are focused on this year, with attention payed on which innovative formula will be a block buster and would there be any market disrupters.
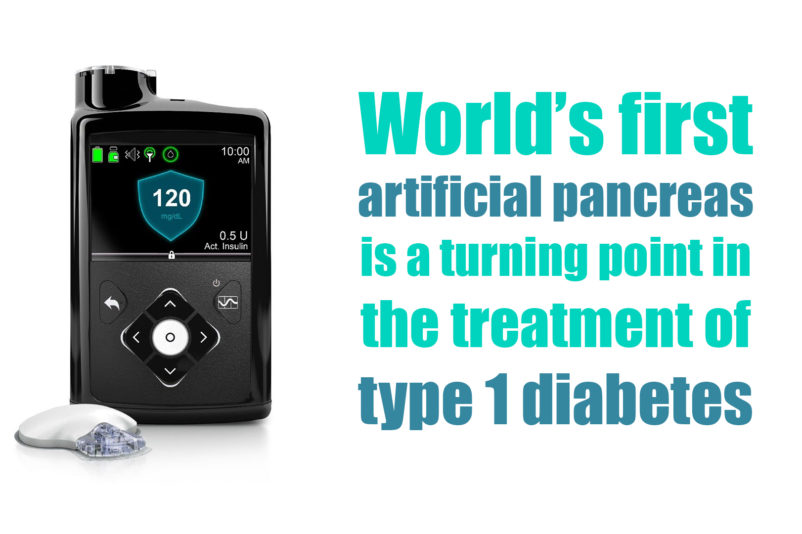
US Food and Drug Administration (FDA) officially announced the approval of the first artificial pancreas for patients with type 1 diabetes. Medtronic’s MiniMed 670G hybrid closed looped system is the first device that automatically tracks the blood glucose (sugar) concentration. Based on the received data it provides the required basal insulin doses. The device is suitable for patients 14 years of age and older, diagnosed with type 1 diabetes. Aspects of...
There is one reliable way to minimize the complexity of the financial status of clinical trials and it is better management with appropriate tools. If you haven’t adopted a clinical trial management system (CTMS) yet, then you might be experiencing certain hindrances in your payment relationships. Chiefly, complicated payments in clinical trials are the result of hitherto prevailing usage of manual systems to manage payments.
Organizations, which still use spreadsheets for trial data collection, seem to increase the complexity behind the studies they conduct. Sadly, the intricacy of the advanced softwares created for data collection, makes many organizations still use spreadsheets. Nonetheless, there are various solutions emerging on the market, which can help resolve this problem. Clinical trial management systems (CTMS) are those innovative software...
CTMS, or clinical trial management system, works in favor of the optimization of clinical studies, since they grow more complex and varied. It is often that scientists and specialists involved in the process face many struggles due to the requirement to perform various tasks with less resources. What is more, it is important that the trial stays time- and
CTMS utilization and implementation in clinical research is of great value for sponsors. In studies they have the sophisticated task to manage budgets and payments correctly and on time. Since this is happening on a regular basis there is an utmost need for a system, which can overview the budget in a fast fashion. Furthermore, clinical trials are expensive and their cost-efficacy is dependent on many factors. Therefore, a comprehensive and lightweight tool like Clinicubes CTMS might prove...
Clinical trial management systems (CTMS) and the industry they are part of have changed drastically. The world they govern will never be the same as they are constantly evolving. Because of the fast-developing research industry, the processes behind any clinical trial become more and more complex. In order to facilitate the whole management of studies and the way specialists conduct their research,...
The health industry could not have its contemporary face without the continuous development of new drugs. The research processes include extensive implementation of clinical trials, which are probably the most vital part of the testing and introduction of new medicaments. One can hardly imagine how much data is involved, and therefore standardizing the processes is of major importance. In the age of technology and online communication the utilization of latest application and computer programs...